Based on structure and functional mechanisms, antibody therapies can be divided into three main forms: monospecific antibodies, bispecific antibodies, and antibodies conjugated with payloads(such as drugs, toxins, or radioisotopes).
01
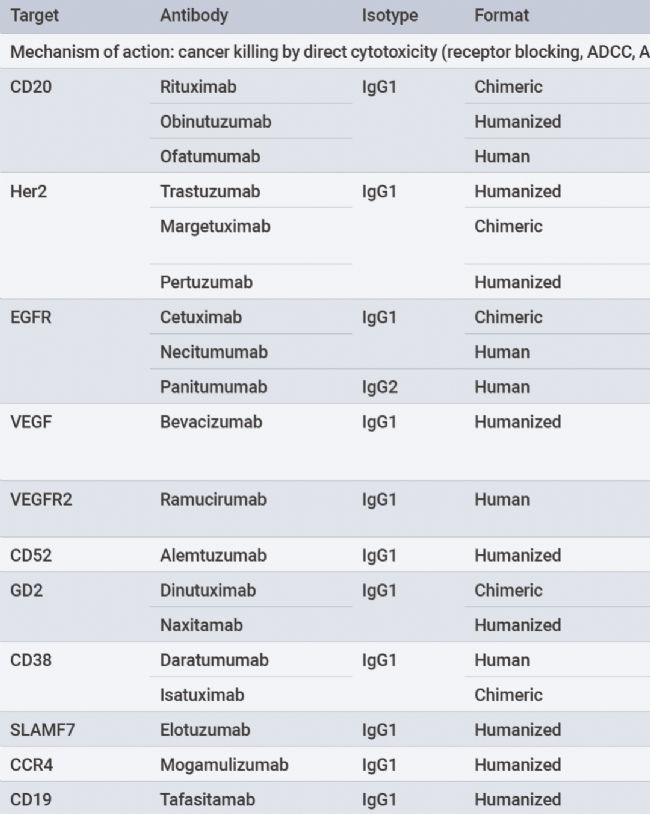
The form of monospecific antibodies involves full-length immunoglobulins that bind to target antigens. Among the five immunoglobulin isotypes(lgG,IgM,IgA,IgE, andIgD), onlylgG binds to the neonatalFc receptor(FcRn), resulting in a long half-life(approximately21days). Cancer-targeting antibodies use theIgG isotype to utilize this extended half-life and are typically administered every21 days. MostFDA-approved andEMA-approved as well as developing antibodies are in the form of monospecificIgG antibodies.TheIgG antibodies are divided into four subtypes(lgG1,IgG2,IgG3, andIgG4), most therapeutic antibodies use thelgG1 subtype.
Table1.FDA andEMA approved or to-be-approved monospecific antibodies(partial)[1].
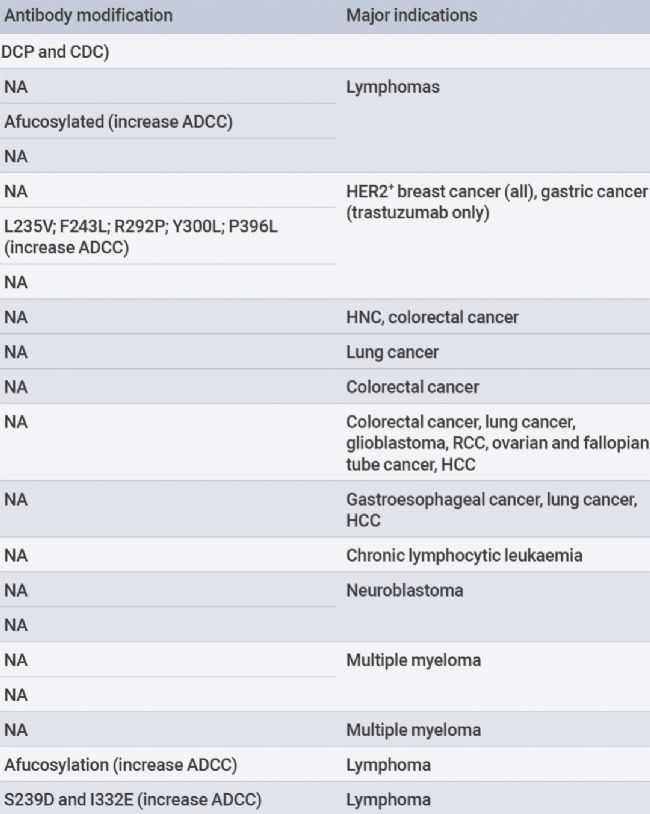
Antibodies bind to cancer cells, leading to their death through various mechanisms. EachIgG antibody subtype provides differentQ effector functions, recruiting and activating immune effector cells to induce cytotoxicity.IgG1 subtypeFc segments bind to activatedFcy receptors(FcyRs,FcyRI,FcyRlla,FcyRlla, andFcyRIIIb) expressed by macrophages and natural killer(NK) cells.FcyR binding leads to macrophage andNK cell-mediated antibody-dependent cellular cytotoxicity(ADCC) and antibody-dependent cellular phagocytosis(ADCP), directly killing cancer cells.
Figure1.Mechanisms of immune activation by monospecific antibodies[1].
02
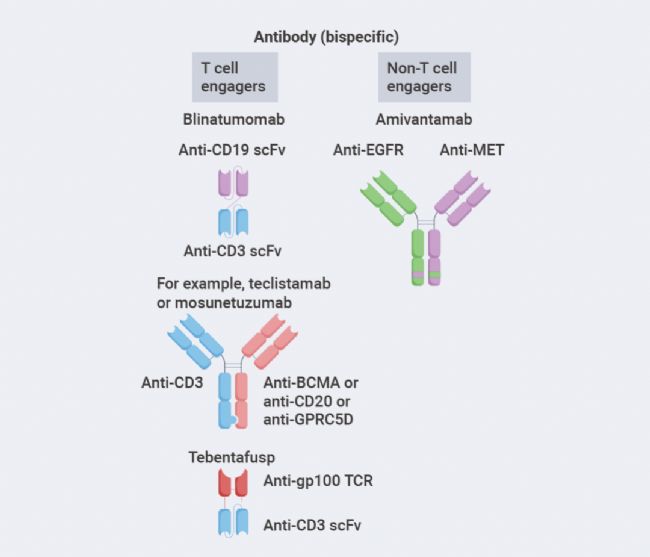
Unlike monospecific antibodies, bispecific antibodies bind to two different antigens or epitopes, which can be located on the same target cell or on different cells. Most bispecific antibodies targeting two different cells areT cell engagers, crosslinking cancer cells with effectorT cells. Thus, they are namedT cell engager(TCE) bispecific antibodies. Upon crosslinking, effectorT cells are activated to kill the bound target cancer cells by releasing cytotoxic granules and lymphokines.
Another class of bispecific antibodies binds to different antigens expressed on the same target cell, such as two different growth factor receptors. These bispecific antibodies kill target cells by blocking proliferative signaling through the target growth factor receptors and by activatingNK cells and macrophages against cancer cells.
Figure2.Classification of bispecific antibodies[1].
03
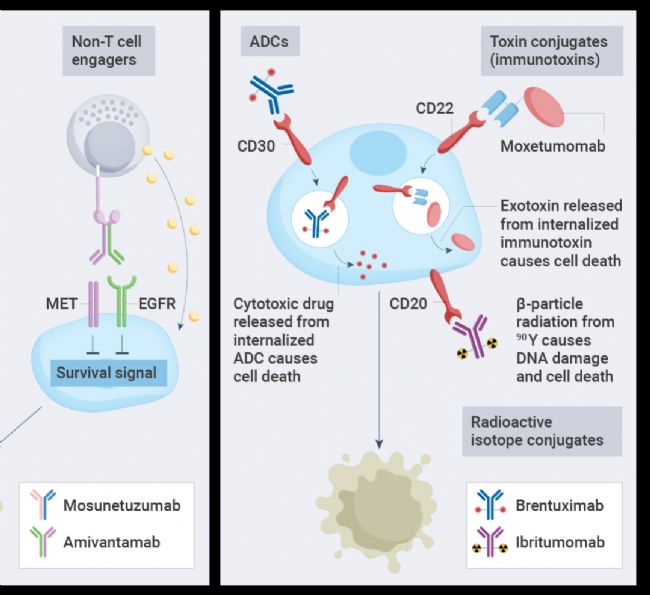
The third main form involves antibodies linked to toxic payloads, such as cytotoxic drugs(antibody-drug conjugates,ADC), bacterial or plant toxins(immunotoxins), or radioisotopes, enhancing the ability of the antibody to kill cancer cells.ADCs are the most widely used form to date, while toxin-conjugated and radioisotope-conjugated antibodies have yet to be widely adopted.
Figure3.Forms of antibody-conjugated drugs[1].
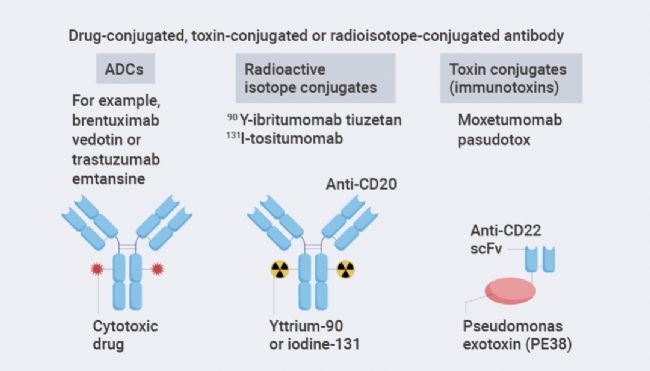
The construction of ADCs is to link a tumor-targeting antibody with a cytotoxic drug, which binds to cell surface antigens leading to internalization, followed by the release of the cytotoxic drug inside the cell. Key components of ADCs include the tumor-targeting antibody, the cytotoxic drug, and the linker that connects the antibody to the cytotoxic drug. ADC success depends on the selection of these key components as well as the conjugation method of connecting the linker to the antibody, which determines the drug-to-antibody ratio(DAR).
Antibody-Radioisotope Conjugates. Antibody-radioisotope conjugates consist of targeted antibodies linked to radioisotopes. Radioisotopes emita particles orβ particles, leading toDNA strand breaks in target cells, causing cell death. Antibody-radioisotope conjugates do not require internalization to induce cell death.
Updated: Dec 30, 2024