Recently, the new mechanism of GP73 in liver injury was published in Nature Communications by Professor Sun Zhiwei and Dr. Gao Qi of Sungen Biomedical R&D team,together with Xijing Hospital of Air Force Military Medical University, the First Affiliated Hospital of Xi 'an Jiaotong University, Huazhong University of Science and Technology, Northwest A&F University, etc. The article titled "Deficiency of ASGR1 promotes liver injury by increasing GP73-mediated hepatic endoplasmic reticulum stress" reveals hepatocellular specific expression of lectin-sialoglycoprotein receptor 1(ASGR1) as a potential genetic determinant of susceptibility to liver injury, and suggests it as the target for treatment of liver injury.

Liver injury is a core pathological process in the majority of liver diseases, yet the genetic factors predisposing individuals to its initiation and progression remain poorly understood. Here we show that asialoglycoprotein receptor 1 (ASGR1), a lectin specifically expressed in the liver, is downregulated in patients with liver fibrosis or cirrhosis and male mice with liver injury. ASGR1 deficiency exacerbates while its overexpression mitigates acetaminophen-induced acute and CCl4-induced chronic liver injuries in male mice. Mechanistically, ASGR1 binds to an endoplasmic reticulum stress mediator GP73 and facilitates its lysosomal degradation. ASGR1 depletion increases circulating GP73 levels and promotes the interaction between GP73 and BIP to activate endoplasmic reticulum stress, leading to liver injury. Neutralization of GP73 not only attenuates ASGR1 deficiency-induced liver injuries but also improves survival in mice received a lethal dose of acetaminophen. Collectively, these findings identify ASGR1 as a potential genetic determinant of susceptibility to liver injury and propose it as a therapeutic target for the treatment of liver injury.
As the result, hepatic ASGR1 is reduced in humans and mice with liver injury. To investigate the potential association between ASGR1 and liver injury, the study determined hepatic ASGR1 expression in patients diagnosed with liver fibrosis or cirrhosis. The results showed significant reductions in both mRNA and protein levels of ASGR1 in these livers compared to normal controls. ASGR1 deficiency accelerates APAP-induced acute liver injury.
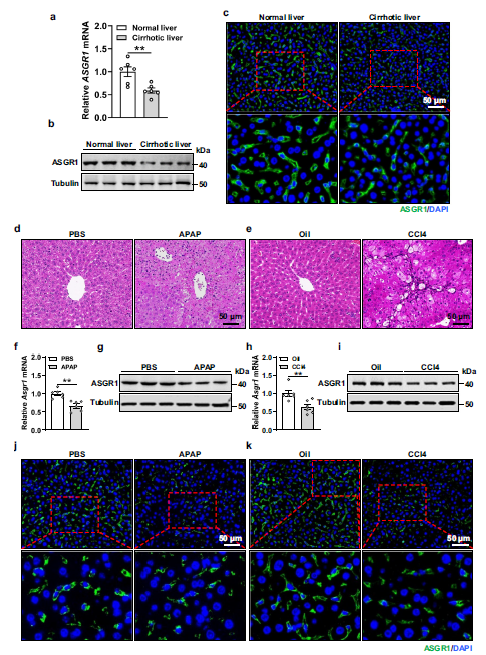
Fig. Hepatic ASGR1 is downregulated in cirrhotic patients and liver injured mice.
a, b Relative mRNA and protein expression of asialoglycoprotein receptor 1 (ASGR1) in normal and cirrhotic human liver tissues (n = 6 per group). c Immunofluorescence staining of ASGR1 in normal and cirrhotic human liver tissues. d–k 8-week-old mice were intraperitoneally injected with either acetaminophen (APAP, 400 mg/kg body weight, a single dose) to induce acute liver injury with PBS as control treatment, or carbon tetrachloride (CCl4, 1 ml/kg body weight, twice a week for 6 weeks) to induce chronic liver injury with oil as control treatment (n = 6 per group). d, e H&E staining of liver sections. Scale bar, 50 μm. f–i Relative mRNA and protein expression of hepatic ASGR1 in mice treated with APAP or CCl4. j, k Representative immunofluorescence staining of ASGR1 in livers of mice treated with APAP or CCl4.
To unravel the mechanism by which anti-GP73 mitigates liver injury in ASGR1-deficient mice, the study assessed hepatic ER stress levels and observed significant reductions in hepatic mRNA and protein levels of BIP and CHOP upon GP73 neutralization in Asgr1−/− mice. They then explored whether GP73 neutralization-induced reduction in ER stress contributes to enhanced liver regeneration and thus alleviates liver injury. Asgr1−/− mice treated with APAP displayed markedly impaired liver regeneration, characterized by downregulated expression of cyclin A2/B1/D1/E1 and a decreased number of Ki67 positive cells when compared to their corresponding WT controls. In contrast, anti-GP73 significantly increased liver regeneration in both genotypes of mice, with a more pronounced effect observed in Asgr1−/− mice treated with APAP. However, when these mice were further treated with the ER stress agonist tunicamycin (Tm), the GP73 neutralization-induced liver regeneration completely disappeared in Asgr1−/− mice, indicating that GP73 neutralization promotes liver regeneration in Asgr1−/− mice by inhibiting hepatic ER stress.
Most importantly, the ASGR1-GP73 axis holds promise as a potential therapeutic target for liver injuries induced by various factors and other related diseases such as COVID-19.
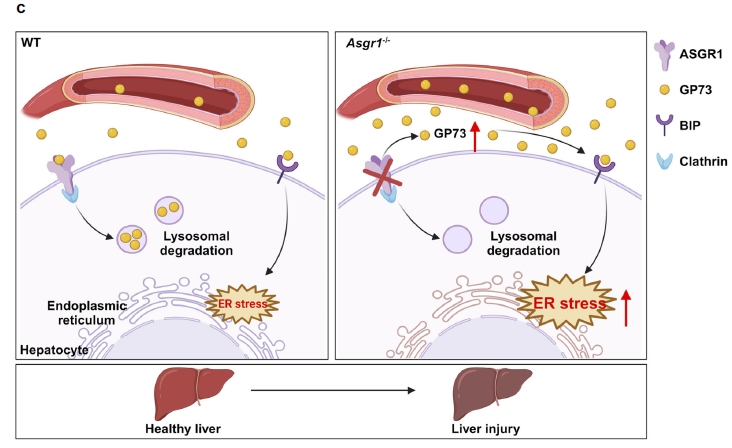
Fig. GP73 neutralization improves survival in ASGR1-deficient mice treated with APAP.
Together, these findings reveal ASGR1 as a candidate underlying genetic predisposition for liver injury, calling special attention to the potential risks associated with ASGR1 inhibition as a therapeutic strategy for the prevention and treatment of CAD.
Updated: Jul 24, 2024