On August 05, 2024, the world's first innovative drug SGC001 injection obtained the clinical trial implied approval from the Center for Drug Evaluation (CDE). The drug is developed by Beijing Sungen Biomedical Technology Co., Ltd. (Sungen Biomedical), an innovative biopharmaceutical company incubated by Beijing Hotgen Biotech Co., Ltd. (SH.688068). This is another milestone following the product's clinical trial (IND) approval from US FDA on May 22, 2024.
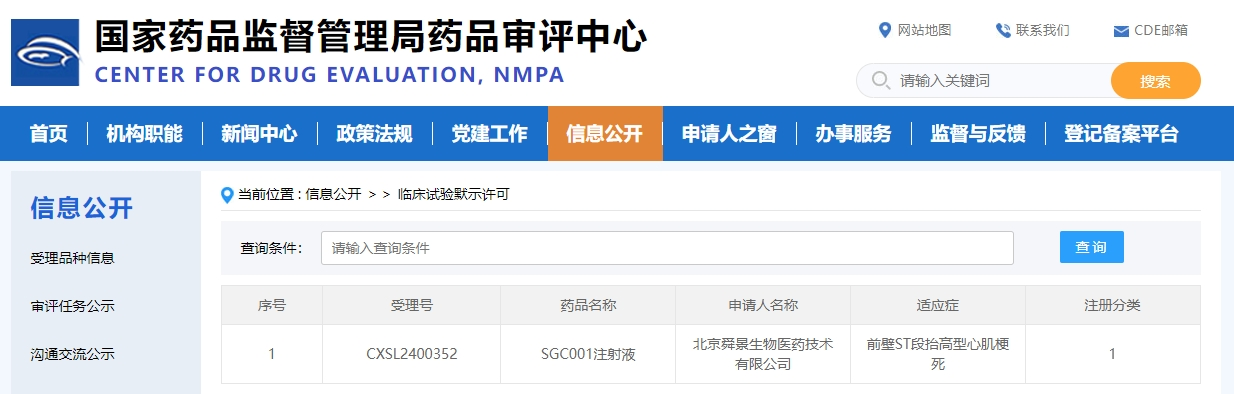
SGC001 is a monoclonal antibody indicated for emergency treatment of acute myocardial infarction (AMI). The drug is developed by Professor Zhiwei Sun's team from Sungen Biomedical, in collaboration with Professor Jie Du's team from Beijing Institute of Heart, Lung and Blood Vessel Diseases, Capital Medical University.
Till now, there is no antibody therapy for AMI approved for clinical or commercial stage. Preclinical pharmacodynamic and toxicological studies reveals that SGC001 has obvious therapeutic effects on heart failure and pathological remodeling of the heart after AMI, with significantly decreased mortality rate, reduced infarction size, improved cardiac functions, good efficacy and a reliable therapeutic window. SGC001 has the potential to become a first-in-class drug, providing safer and more effective therapies for AMI patients worldwide.
Updated: Aug 09, 2024